-
PDF
- Split View
-
Views
-
Cite
Cite
Scott H. Stewart, Adrian Reuben, Walter A. Brzezinski, David G. Koch, Jan Basile, Patrick K. Randall, Peter M. Miller, Preliminary Evaluation of Phosphatidylethanol and Alcohol Consumption in Patients with Liver Disease and Hypertension, Alcohol and Alcoholism, Volume 44, Issue 5, September-October 2009, Pages 464–467, https://doi.org/10.1093/alcalc/agp039
Close - Share Icon Share
Abstract
Aims: The goal of this preliminary study was to evaluate the relationship between blood phosphatidylethanol (PEth) and recent drinking in patients with liver disease and hypertension. Methods: Twenty-one patients with liver disease and 21 patients with essential hypertension were recruited at an academic medical center. Alcohol consumption was estimated using validated self-report methods, and blood PEth was measured by HPLC-MS/MS at a contracted laboratory. Nonparametric comparisons were made between abstainers/light drinkers, moderate drinkers consuming between 1 and 3 drinks per day, and those drinking above this level. Regression methods were used to estimate the effects of liver disease, gender, and age on the relationship between PEth and alcohol use, and to estimate the strength of the linear relationship between PEth and drinking. Results: PEth differed significantly between the three drinking groups (P < 0.001). The relationship between PEth and alcohol did not differ between hypertension and liver disease patients (P = 0.696), nor by gender and age. While there was substantial variability between subjects in the PEth concentration given a similar level of reported drinking, the amount of ethanol consumed was strongly associated with the PEth concentration (P < 0.001). Conclusion: Results support PEth measurement by HPLC-MS/MS as a promising marker of past 1- to 2-week moderate to heavy alcohol consumption in patients with and without liver disease. PEth appears useful for differentiating abstinence or light drinking from moderate to heavy consumption, but may have limited utility for differentiating moderate from heavy alcohol use.
INTRODUCTION
Chronic heavy drinking is a direct cause of many medical conditions due to toxic effects of ethanol or its metabolism (Saitz, 2003), and effective treatment requires abstinence or a sharp reduction in drinking. Assessment of alcohol consumption in clinical and research settings mainly relies on self-report (National Institute on Alcohol Abuse and Alcoholism, 2007). While very useful, self-report methods are in general subject to potential sources of bias (Babor et al., 2000; Del Boca and Darkes, 2003), which may be magnified in patients with alcohol-associated illnesses. Such individuals may wish to conceal their heavy drinking or prior unsuccessful attempts to control their drinking due to associated stigma, and may suffer from cognitive impairment due to chronic alcohol abuse or other factors.
Biomarkers of alcohol consumption may aid detection and treatment efforts in patients with alcohol-related conditions (Allen, 2003) by providing highly objective indicators of recent ethanol exposure. However, biomarkers typically do not have adequate sensitivity and specificity in medical populations, particularly with liver disease, where other factors besides alcohol can influence markers such as gamma glutamyltransferase, aminotransferases and red blood cell mean corpuscular volume (Conigrave et al., 2003). The only FDA-approved marker for heavy drinking is the percent carbohydrate-deficient transferrin (Anton, 2001), but this requires ∼60 g of ethanol per day (i.e. 4–5 standard drinks) to become elevated, has a sensitivity of 60–70%, and loses its otherwise very high specificity in advanced liver disease (Heinemann et al., 1998; DiMartini et al., 2001). Blood phosphatidylethanol (PEth) is a minor, non-oxidative product of ethanol elimination that is formed from phosphatidylcholine and ethanol via the action of phospholipase D (Gustavsson, 1995), which can occur extra-hepatically such as in human erythrocytes (Butikofer et al., 1993). Given this mechanism of formation, the relationship between PEth and drinking may be less influenced by liver function relative to other biomarkers. Circulating PEth is found primarily in the red blood cell fraction (Varga et al., 2000), has a detection window of ∼1–3 weeks following drinking cessation and seems highly sensitive and specific in differentiating known heavy drinkers from known social drinkers and abstainers when measured using an evaporative light scattering detector assay (Hartmann et al., 2007). This PEth assay has been used, for example, to detect unreported drinking in emergency department patients (Kip et al., 2008). An alternative mass spectrometer-based assay may be more sensitive for detecting low PEth concentrations (Gunnarsson et al., 1998) and may allow for the detection of even moderate consumption. As such, the latter assay may reveal less heavy but still harmful drinking in patients with alcohol-associated disorders, and may also predict the amount of alcohol consumed. However, prior to use as a marker of alcohol consumption in clinical care or clinical research, the relationship between PEth and drinking must be characterized in relevant populations that show a wide range of recent alcohol exposure. This manuscript describes our pilot testing of PEth in patients with two conditions that can be caused or aggravated by frequent moderate to heavy drinking, namely liver disease and hypertension. Importantly, liver impairment, by influencing the major oxidative mechanisms for ethanol elimination that occur primarily in the liver, may modify the association between the amount of alcohol consumed and PEth. Since hypertension should not modify this association, hypertension patients were also viewed as a control group for exploring our hypothesis that PEth synthesis may be related to liver function.
METHODS
Subjects and recruitment strategy
We recruited 21 consecutively eligible and consenting patients with liver disease from inpatient and outpatient hepatology services, aiming to include a minimum of 10 subjects with recent drinking. The main exclusion criterion was cognitive dysfunction of sufficient severity to preclude informed consent. The clinically diagnosed etiology, alcohol or otherwise, was confirmed by a review of the medical record with the attending hepatologists who were responsible for the liver disease patients, and the severity of liver disease was estimated using the Model for End-Stage Liver Disease (MELD) (Kamath et al., 2001). The MELD score is calculated from the international normalized ratio (INR), creatinine and total bilirubin, and is used in the United States for prioritizing liver transplantation. In order to compare the PEth–drinking relationship in a group without liver disease and to assess the potential of PEth as a screen for alcohol-related hypertension, we recruited an identical number of hypertension patients. Because differentiating abstinence from light to moderate alcohol use is not of concern in hypertension, this sample was limited to current drinkers, defined as those who reported having at least one drink per week. Potential subjects were asked to participate in a study that included blood tests and a survey about their alcohol drinking. Those who provided signed informed consent were enrolled and interviewed, and received $25 compensation following completion. The protocols were approved by the university Institutional Review Board (federal-wide assurance number for protection of human subjects 00001888).
Alcohol consumption data
Alcohol consumption for the month prior to enrollment was estimated by personnel with clinical alcohol research experience using a timeline follow-back method (Sobell and Sobell, 1992). This approach uses typical drinking habits and memory cues to obtain daily drinking estimates. The estimated quantity of alcoholic beverage consumed in US fluid ounces was converted to milliliters, and grams of ethanol were calculated from the ethanol content of the beverages and the density of ethanol (i.e., 0.79 g/ml). In presenting the results of this study, we defined one standard drink as 14 g of ethanol (equivalent, for example, to a 12-oz beer containing 5% ethanol, or a 1.5-oz serving of 80 proof spirits).
Collection and measurement of blood PEth
Ten mL of whole blood was drawn into a tube containing 100 mg sodium fluoride and 20 mg potassium oxalate. Samples were shipped fresh or following a maximum of 2 weeks storage at −20°C to a contracted laboratory (US Drug Testing Laboratories, Des Plaines, IL, USA) that was masked to the status of the study subjects. The HPLC-MS/MS assay detected the isomers 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphatidylethanol and 1-oleoyl-2-palmitoyl-sn-glycero-3-phosphatidylethanol, which included ∼40% of total PEth (Gunnarsson et al., 1998). The within-batch coefficient of variation for the assay was 9%, the between-batch coefficient of variation 13% and the statistical limit of quantification 19.8 ng/ml. A test was considered positive at 20 ng/ml or higher (i.e. above the assay cutoff at which PEth can reliably be detected over background noise).
Data analysis
Data analysis included non-parametric methods for PEth comparison between subjects reporting abstinence or an average of less than one drink per day (n = 17), those reporting an average of one to three drinks per day (n = 14) and those reporting more than three drinks per day during the prior 2 weeks (n = 11). Pairwise comparisons were made without adjustment for multiple comparisons due to the preliminary nature of this work. Regression methods were also used to further describe the relationship between PEth and drinking, and to explore for any modification of PEth synthesis by diagnosis (i.e., liver disease versus hypertension), gender and age (dichotomized at the median of 52). The PEth concentration served as the dependent variable, and estimated grams of alcohol consumed in the 2 weeks preceding measurement was the main independent variable. We added first-order interaction terms for alcohol consumption with liver disease, age (dichomtomized at the median) and gender to explore modification of the PEth–alcohol relationship by these factors. The distribution of PEth included a number of censored results (i.e. PEth was not quantifiable between 0 and 20 ng/ml). Because of this distribution, we used tobit rather than linear regression, which includes adjustment for the unknown distribution of PEth below the limit of quantitation (Austin et al., 2000).
RESULTS
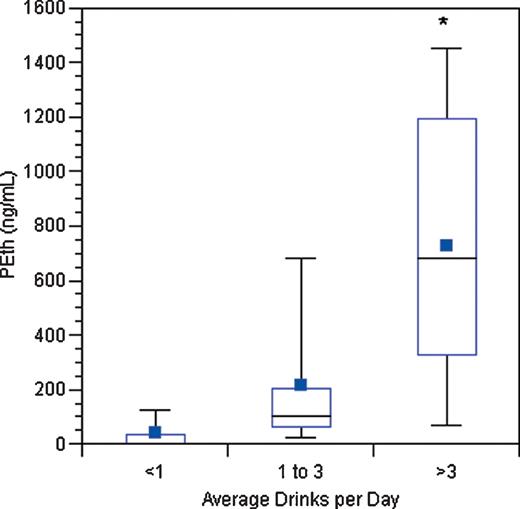
Descriptive characteristics of the subjects are listed in Table 1. The overall median age was 52, about two-thirds were male and 70% had quantifiable PEth. For the liver disease patients, two-thirds had a diagnosis of alcoholic liver disease, and the MELD scores indicate inclusion of patients with both mildly and severely impaired liver function. Box plots of the PEth results for subjects in each drinking category are shown in Fig. 1. There were 17 subjects in the abstinent to less than one drink per day group (11 liver disease subjects and 6 hypertension subjects), 14 in the group averaging between one and three drinks per day (4 liver disease subjects and 10 hypertension subjects) and 11 in the group averaging more than three drinks per day (6 liver disease subjects and 5 hypertension subjects).
Box plot of PEth concentrations by drinking category (tails indicate the range, horizontal lines indicate the median and interquartile range and solid squares indicate the mean). The asterisk in the plot for the heaviest drinking group indicates that one outlying PEth value of 3710 ng/ml was truncated to 1500 ng/ml to facilitate presentation of the results. The overall comparison (P < 0.001) and each pairwise comparison (P < 0.05) were statistically significant.
Subject characteristics
| Characteristic . | Liver disease patients (n = 21) . | Hypertension patients (n = 21) . |
|---|---|---|
| Median age (range) | 50 (33–64) | 60 (44–74) |
| Male (%) | 62 | 71 |
| Non-Hispanic white (%) | 71 | 57 |
| Current drinkers (%) | 57 | 100 |
| Median grams ethanol in past 2 weeks (interquartile range; range) | 165 (0–726; 0–2816) | 374 (141–539; 45–1602) |
| Quantifiable Peth (%) | 62 | 76 |
| Median PEth (ng/ml) (interquartile range; range) | 63 (0–431; 0–3710) | 86 (28–206; 0–1016) |
| Median MELD score (range)a | 16 (6–32) | N/A |
| Clinical diagnosis of alcoholic liver diseaseb (%) | 66 | N/A |
| Characteristic . | Liver disease patients (n = 21) . | Hypertension patients (n = 21) . |
|---|---|---|
| Median age (range) | 50 (33–64) | 60 (44–74) |
| Male (%) | 62 | 71 |
| Non-Hispanic white (%) | 71 | 57 |
| Current drinkers (%) | 57 | 100 |
| Median grams ethanol in past 2 weeks (interquartile range; range) | 165 (0–726; 0–2816) | 374 (141–539; 45–1602) |
| Quantifiable Peth (%) | 62 | 76 |
| Median PEth (ng/ml) (interquartile range; range) | 63 (0–431; 0–3710) | 86 (28–206; 0–1016) |
| Median MELD score (range)a | 16 (6–32) | N/A |
| Clinical diagnosis of alcoholic liver diseaseb (%) | 66 | N/A |
aMELD indicates the Model for End-Stage Liver Disease severity score.
bThe 14 patients with alcoholic liver disease include 5 patients with co-morbid chronic Hepatitis C.
Subject characteristics
| Characteristic . | Liver disease patients (n = 21) . | Hypertension patients (n = 21) . |
|---|---|---|
| Median age (range) | 50 (33–64) | 60 (44–74) |
| Male (%) | 62 | 71 |
| Non-Hispanic white (%) | 71 | 57 |
| Current drinkers (%) | 57 | 100 |
| Median grams ethanol in past 2 weeks (interquartile range; range) | 165 (0–726; 0–2816) | 374 (141–539; 45–1602) |
| Quantifiable Peth (%) | 62 | 76 |
| Median PEth (ng/ml) (interquartile range; range) | 63 (0–431; 0–3710) | 86 (28–206; 0–1016) |
| Median MELD score (range)a | 16 (6–32) | N/A |
| Clinical diagnosis of alcoholic liver diseaseb (%) | 66 | N/A |
| Characteristic . | Liver disease patients (n = 21) . | Hypertension patients (n = 21) . |
|---|---|---|
| Median age (range) | 50 (33–64) | 60 (44–74) |
| Male (%) | 62 | 71 |
| Non-Hispanic white (%) | 71 | 57 |
| Current drinkers (%) | 57 | 100 |
| Median grams ethanol in past 2 weeks (interquartile range; range) | 165 (0–726; 0–2816) | 374 (141–539; 45–1602) |
| Quantifiable Peth (%) | 62 | 76 |
| Median PEth (ng/ml) (interquartile range; range) | 63 (0–431; 0–3710) | 86 (28–206; 0–1016) |
| Median MELD score (range)a | 16 (6–32) | N/A |
| Clinical diagnosis of alcoholic liver diseaseb (%) | 66 | N/A |
aMELD indicates the Model for End-Stage Liver Disease severity score.
bThe 14 patients with alcoholic liver disease include 5 patients with co-morbid chronic Hepatitis C.
Despite the inclusion of two alcoholic liver disease subjects who reported at least 2 months of abstinence but had quantifiable PEth (specifically, 44 ng/ml and 427 ng/ml), there was a statistically significant difference in PEth findings between the three groups (Kruskal–Wallis P < 0.001, see Fig. 1). Pairwise comparisons demonstrated significant differences between those consuming less than one drink per day versus those consuming one to three drinks per day (P = 0.002), those consuming less than one drink per day versus those consuming more than three drinks per day (P < 0.001) and those consuming one to three drinks per day versus those consuming more than three drinks per day (P = 0.020). Spearman's correlations between alcohol consumption and PEth were 0.76 (P < 0.001) for liver disease subjects and 0.66 (P = 0.001) for hypertension subjects. Consistent with these similar correlations, the Tobit regression also suggested that the relationship between drinking and PEth concentration was comparable in the liver disease and hypertension subjects (P-value for interaction = 0.696), as well as similar for men and women (P = 0.221), and unaffected by age (P = 0.591). There was a statistically significant association between PEth and grams of ethanol consumed in the prior 2 weeks (regression slope = 0.81, P < 0.001). Regression results were repeated without an outlying observation having a PEth of 3710 ng/ml, resulting in a regression slope = 0.54 (P < 0.001).
DISCUSSION
This preliminary study suggested that blood PEth predicted alcohol consumption in patients with liver disease and hypertension, and that the effect of alcohol consumption on the PEth concentration is not dependent on liver function. Higher PEth concentrations predicted greater alcohol consumption, but there was substantial variability between individuals. This suggests that a single PEth measurement is useful for detecting recent drinking but is a crude estimate of the amount of alcohol consumed.
Early phase validation studies primarily involving heavy drinkers in detoxification facilities have mainly utilized a different PEth assay (Hansson et al., 1997; Aradottir et al., 2006; Hartmann et al., 2007). With an evaporative light-scattering detection system, PEth has been ∼95% sensitive and 100% specific in differentiating very heavy drinkers from known abstainers and social drinkers. Our results suggest that PEth quantification using the mass spectrometer-based assay will detect even moderate consumption in patients with potentially alcohol-associated disorders. However, due to the apparent variability in PEth synthesis between individuals, specificity may be limited for differentiating moderate from heavy drinking. Regarding specificity for any ethanol ingestion, we did find quantifiable PEth in two self-reported abstainers with alcoholic liver disease. It is possible that these represented false positive PEth results. However, since ethanol is required for PEth synthesis, these cases likely represent an underestimation of actual alcohol ingestion and thus biomarker detection of recent drinking that was missed by detailed self-report methods. Supporting this, no non-alcoholic liver disease patients who reported abstinence had detectable PEth, and the lowest reported consumption resulting in PEth detection among the hypertension subjects was equivalent to about one drink per day.
The main strengths of this report are the evaluation of a novel alcohol consumption biomarker in medical patients with conditions that can be caused by alcohol consumption, the comparison between patients with and without liver disease, and the representation of both light to moderate and heavier drinkers. In situations where highly objective estimates of alcohol drinking are particularly desirable (e.g. liver transplant evaluations, clinical research protocols, confirmation of alcohol use in both acute and chronic alcohol-related illness), PEth may be useful in determining the presence or absence of at least moderate consumption. PEth appears particularly useful in patients with liver disease, in whom many alcohol biomarkers (e.g. aminotransferases, gamma-glutamyltransferase, mean red blood cell volume, percent carbohydrate-deficient transferrin) may be elevated due to the liver disease itself (Conigrave et al., 2003). PEth also appears sensitive for moderate drinking, with many of the aforementioned markers only responding to heavy drinking. Conversely, unlike some other ethanol metabolites such as ethyl glucuronide in the urine, minimal drinking or unintentional ethanol ingestion will likely not result in detectable PEth (Palmer, 2009). Given the preliminary nature of this work, future studies will need to confirm these findings in larger samples, evaluate the effects of drinking patterns (e.g. binge episodes, abstinent days prior to measurement) on PEth concentration and identify sources of variation in PEth other than recent consumption, such as a history of chronic heavy drinking (Varga and Alling, 2002). For widespread clinical use, it will also be important to develop and validate assays that will be practical for typical clinical laboratories. The recent development of monoclonal antibodies to PEth suggests that this will be feasible (Nissinen et al., 2008).
In summary, this pilot study and previously published research suggests that PEth is a highly promising marker of alcohol consumption during the days and weeks preceding measurement. Relative to many other biomarkers, the modest drinking threshold required for mass spectrometry detection, the half-life of ∼4 days, and the probable validity despite impaired liver function suggest unique or complementary strengths for assessing drinking in patients with alcohol-associated conditions.
This work was supported by a Career Development Award from the National Institute on Alcohol Abuse and Alcoholism (K23AA014188).